Molnupiravir | I5stcldw 71 M
Molnupiravir is a shape-shifter called a tautomer. Molnupiravir is a tablet that can be taken at home.
Molnupiravir works as an antiviral agent by inhibiting the replication of the SARS-CoV-2 virus the causative agent of COVID-19.

Molnupiravir. Molnupiravir was generally well tolerated with similar numbers of adverse events across all groups. Molnupiravir is an orally bioavailable form of a potent ribonucleoside analog that inhibits replicating multiple RNA viruses including SARS-CoV-2 the causative agent of COVID-19. What is Molnupiravir.
Molnupiravir has been shown to be active in several. Molnupiravir is a prodrug derivatized from the ribonucleoside analog β-d-N 4-hydroxycytidine NHC that is converted to its active form molnupiravir triphosphate MTP in the cell 6Both Gordon. When it enters the cell it is converted into RNA-like building blocks.
It prompts errors in the viral RNA grouping stopping the viral replication lessening the contamination and restricting infection transmission during the viral RNA replication. Listing a study does not mean it has been evaluated by the US. Efficacy and Safety of Molnupiravir MK-4482 in Hospitalized Adult Participants With COVID-19 MK-4482-001 The safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
Molnupiravir MK-4482 is designed to induce viral genome copying errors to prevent the virus from replicating in the human body and evidence to date from clinical trials in patients with COVID-19 suggests that molnupiravir may reduce replication of the SAR-CoV-2 virus. The multi-centre randomised double-blind placebo-controlled Phase III trial will assess the efficacy and safety of molnupiravir versus placebo in. Merck Pharmaceuticals and Ridgeback Biotherapeutics have announced that their investigational oral therapeutic for the treatment of mild-to-moderate COVID-19 molnupiravir has showed promising results as part of their phase 23 trial.
Molnupiravir drug is an orally effective prodrug of the manufactured nucleoside evolved N4-hydroxycytidine. Molnupiravir is an oral ribonucleoside analog that inhibits RNA virus replication. The drug has certain mutagenic characteristics whereby human cells can also be targeted so there is a theoretical potential for causing genetic alterations or potentially cancers noted research professor Dr Luis Menendez Arias at Consejo Superior de.
Its a very clever drug too. Molnupiravir is an experimental antiviral drug that is orally active and was originally developed for the treatment of influenza. Molnupiravir is an orally available antiviral drug candidate currently in phase III trials for the treatment of patients with COVID-19.
An experimental antiviral therapy molnupiravir is an oral form of a potent ribonucleoside analogue that hinders the replication of various ribonucleic acid RNA viruses such as SARS-CoV-2. Molnupiravir has been shown to be active in several models of SARS-CoV-2 including for prophylaxis treatment and. Molnupiravir was under development before the pandemic began as a potential treatment for flu and other viruses.
It assumes two forms one which closely resembles uracil and the other cytosine. At day 5 there was a reduction nominal p0001 not controlled for multiplicity in positive viral culture in subjects who received molnupiravir all doses compared to. Molnupiravir increases the frequency of viral RNA mutations and impairs SARS-CoV-2 replication in animal models and in humans.
A drug like molnupiravir the name is a reference to Thors hammer Mjölnir could also help compensate for persistent gaps in Covid-19 vaccination coverage both in the United States and abroad. Molnupiravir is an orally available drug which becomes activated through metabolization in the body. In the first phase the.
Molnupiravir MK-4482 Antiviral Description. Merck known as MSD developed molnupiravir in collaboration with. Molnupiravir is the first oral direct-acting antiviral shown to be highly effective at reducing nasopharyngeal SARS-CoV-2 infectious virus and viral RNA and has a favorable safety and tolerability profile.
The drug looks enough like some of the natural building blocks that the. Molnupiravir works by confusing SARS-CoV-2s polymerase the enzyme that builds the viral genome during replication. Molnupiravir EIDD-2801MK-4482 is an investigational orally bioavailable form of a potent ribonucleoside analog in development for the treatment of COVID-19.
2 Molecular echanism of molnupiravir-induced SARS-CoV-2 mutagenesis. The idea is that molnupiravir could be taken as an oral pill by symptomatic patients who test positive for COVID-19 before their illness is severe enough to require going to a. Molnupiravir MK-4482 EIDD-2801 is an experimental oral antiviral developed initially to treat influenza at Emory University.
Results from the trial were also recently presented at the European Congress of Clinical Microbiology and Infectious Diseases ECCMID. Molnupiravir EIDD-2801MK-4482 is an investigational orally bioavailable form of a potent ribonucleoside analog that inhibits the replication of multiple RNA viruses including SARS-CoV-2 the causative agent of COVID-19. Molnupiravir is an orally available antiviral drug candidate currently in phase III trials for the treatment of patients with COVID-19.

Molecular Mechanisms Of Corona Drug Candidate Molnupiravir Unraveled Max Planck Gesellschaft

Antivirale Therapie Positives Und Negatives Zu Molnupiravir Bei Covid 19 Pz Pharmazeutische Zeitung

Weniger Schwere Krankheitsverlaufe Corona Medikament Weckt Hoffnung Tagesschau De
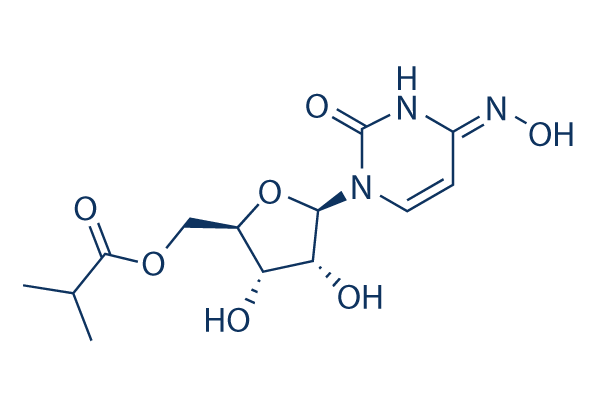
Molnupiravir Eidd 2801 99 Hplc Selleck Sars Cov Inhibitor

A Daily Pill To Treat Covid Could Be Just Months Away Scientists Say

Neue Studiendaten Molnupiravir Schutzt Mause Vor Corona Infektion Und Cov Pz Pharmazeutische Zeitung
Lung Center Qmmc Call For Participants In Molnupiravir Trials Vs Covid 19